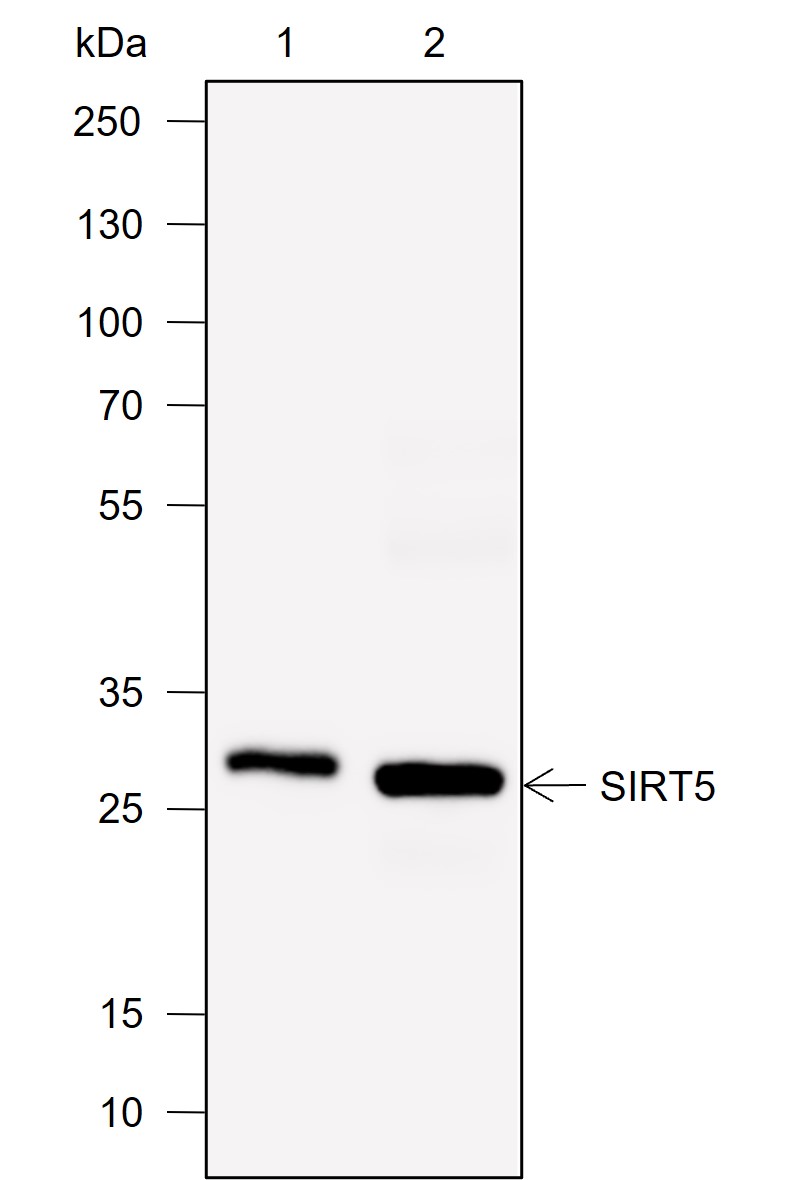
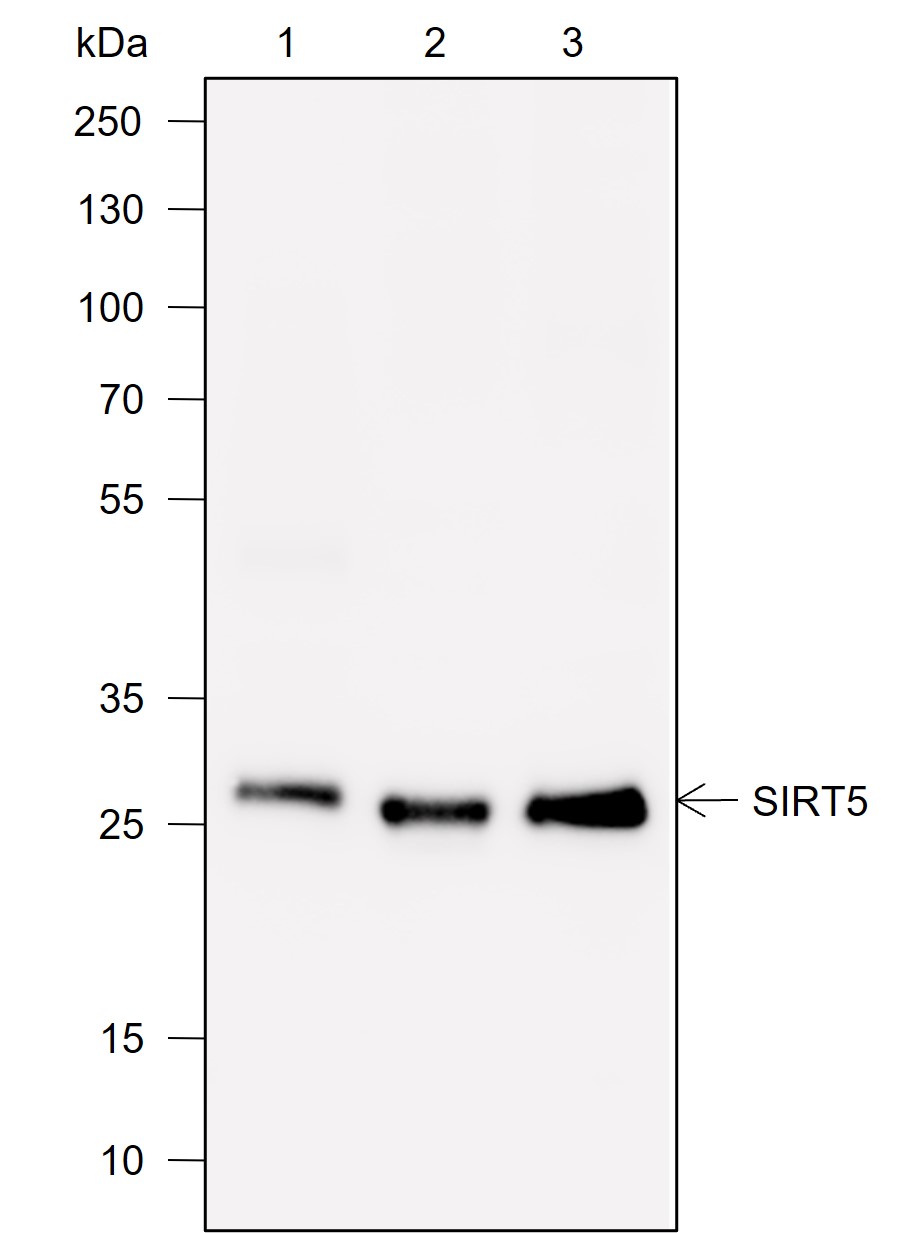
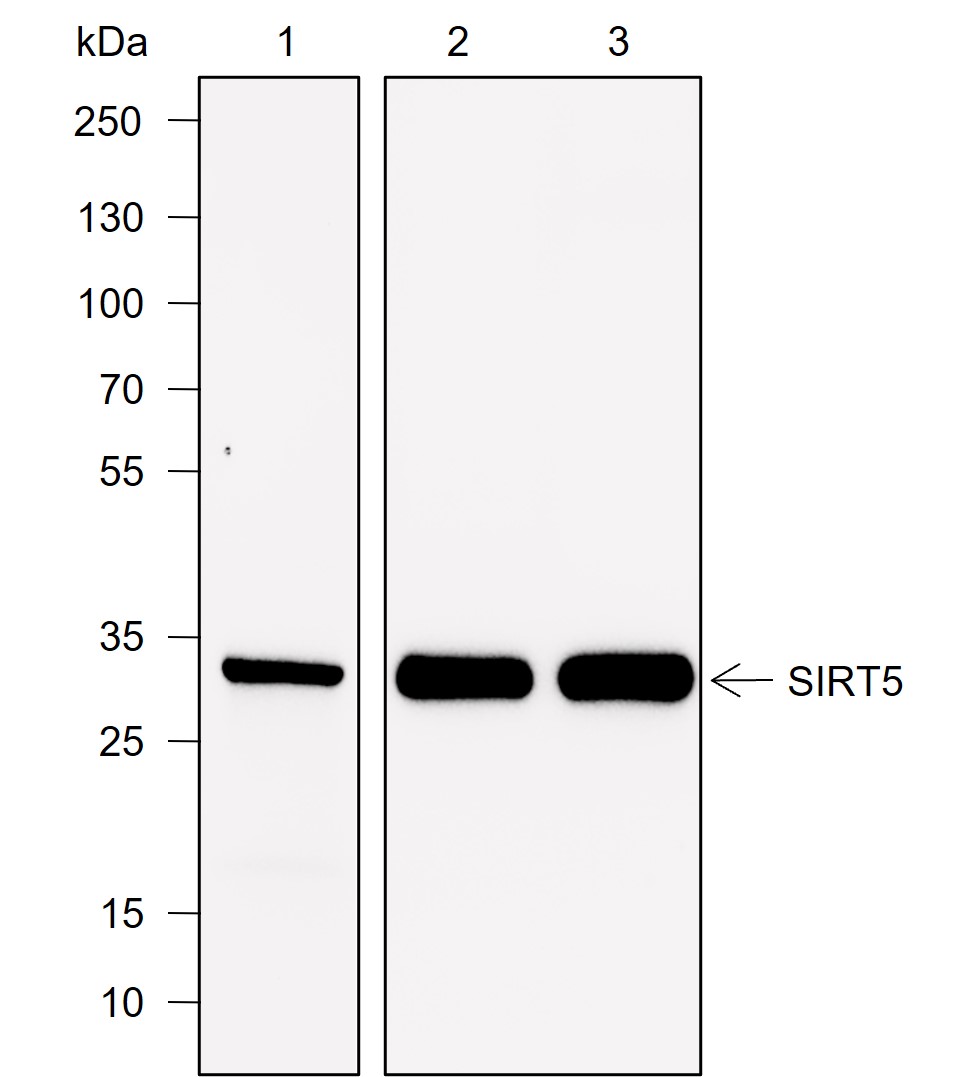
Background
SirT5, a mammalian homolog of Sir2, is localized to the mitochondria and has been implicated in the regulation of cell metabolism. SirT5 deacetylates carbamoyl phosphate synthetase 1 (CPS1) in the mitochondrial matrix and increases its activity in response to fasting, allowing for adaptation to increased amino acid catabolism. SirT5 has also been shown to deacetylate cytochrome c in the mitochondrial intermembrane space. In addition to its deacetylase activity, SirT5 contains lysine desuccinylase and demalonylase activity. Succinyl-lysine and malonyl-lysine modifications occur in a variety of organisms and these post-translational modifications are found on many metabolic enzymes. SirT5 knockout mice show increased levels of succinyl-lysine and malonyl-lysine protein modifications in the liver, including increased succinylation of CPS1, a known target of SirT5, suggesting that SirT5 functions to regulate metabolic enzymes through its deacetylase, desuccinylase, and demalonylase activities.
Cellular location
Mitochondrion, Cytoplasm, Nucleus