Background
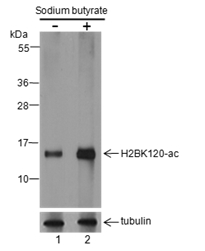
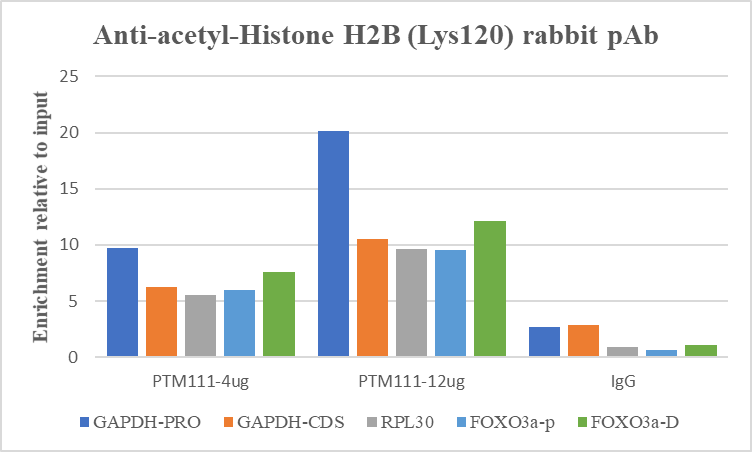
Histone post-translational modifications (PTMs), known as the “histone code”, are key mechanisms of epigenetics that modulate chromatin structures. The PTMs on histone including acetylation, methylation, phosphorylation, and novel acylations directly affect the accessibility of chromatin to transcription factors and other epigenetic regulators, altering genome stability and gene transcription. Histone acetylation is a dynamic PTM tightly controlled by the opposing action of histone acetyltransferases (HATs) and histone deacetylases (HDACs). While acetylation frequently occurs on the N-terminal tails of histones, the acetylation of histone H2B at Lys120 (H2BK120ac) is a distinct mark located within the globular domain. H2BK120ac is strongly associated with active, cell-type-specific enhancers and is recognized as a key mark of transcriptional elongation.
Cellular location
Nucleus